Electron Groups Could Be Considered Which of the Following
For NF_3 the Lewis Structure will give you something like Nitrogen in the center with 3 bonds to F atoms and 1 lone pairI dont know how to draw structures on here. Electron groups are lone pairs andor bonds since we explain bonds as a pair of shared electrons.
10 3 Vsper Theory The Effect Of Lone Pairs Chemistry Libretexts
But 4s subshell is located at greater radius than 3d due to greater size of 4s subshell Which of the the electrons should be considered the last electron.
. Which of the following is considered a single electron group. A triple bond 2. The electron configuration of the vertical columns are similar because they are in the same group meaning that they will have the same groups s p d f and because of that there configurations.
Memorize flashcards and build a practice test to quiz yourself before your exam. Up to 24 cash back An electron configuration is a method of indicating the arrangement of electrons about a nucleus. An electron-donating group adjacent to NH2 or OH makes the rate very low.
Closer to F because fluorine has a higher electronegativity than carbon d. Which of the following is considered a single electron group. What is the angle between electron groups in the trigonal planar electron group.
Question 1 Which of the following is considered an electron group to determine the electron group cometry. A single bond 5 All of the above. In Electronic Configuration electrons are arranged in various shells Subshell and Orbital by following certain rules.
What is the angle between electron groups in the linear election geometry. The two or more electrons can be bonded by single bond double bond covalent bond of electrons can simply be lone pair of electrons. Today I came across a problem which asked some quantum number informations regarding the last electron of ZincNow as far as I have studied 4s subshell is filled in preference to 3d subshell bcos of lower energyof 4s subshell.
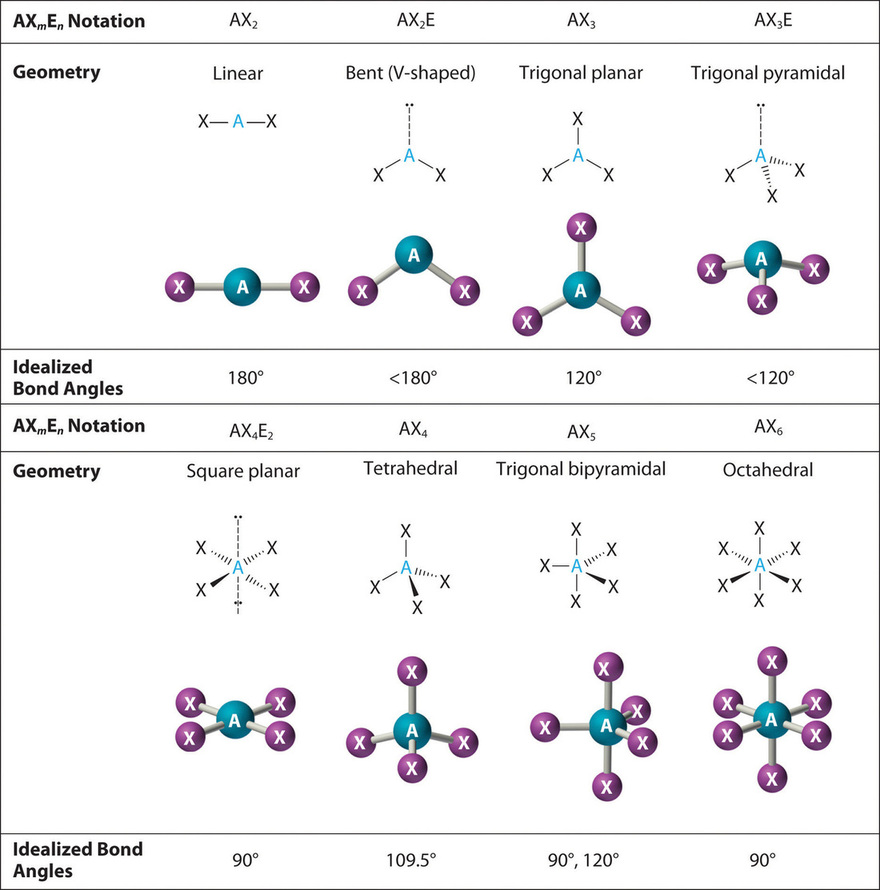
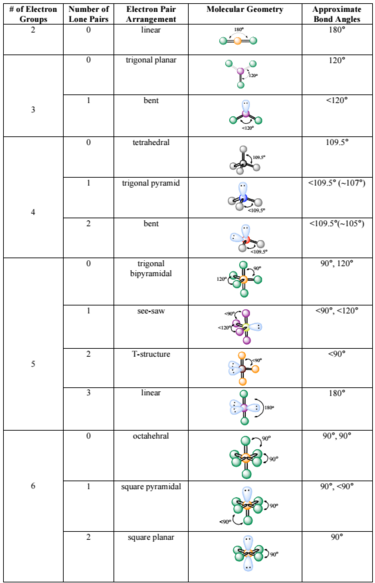
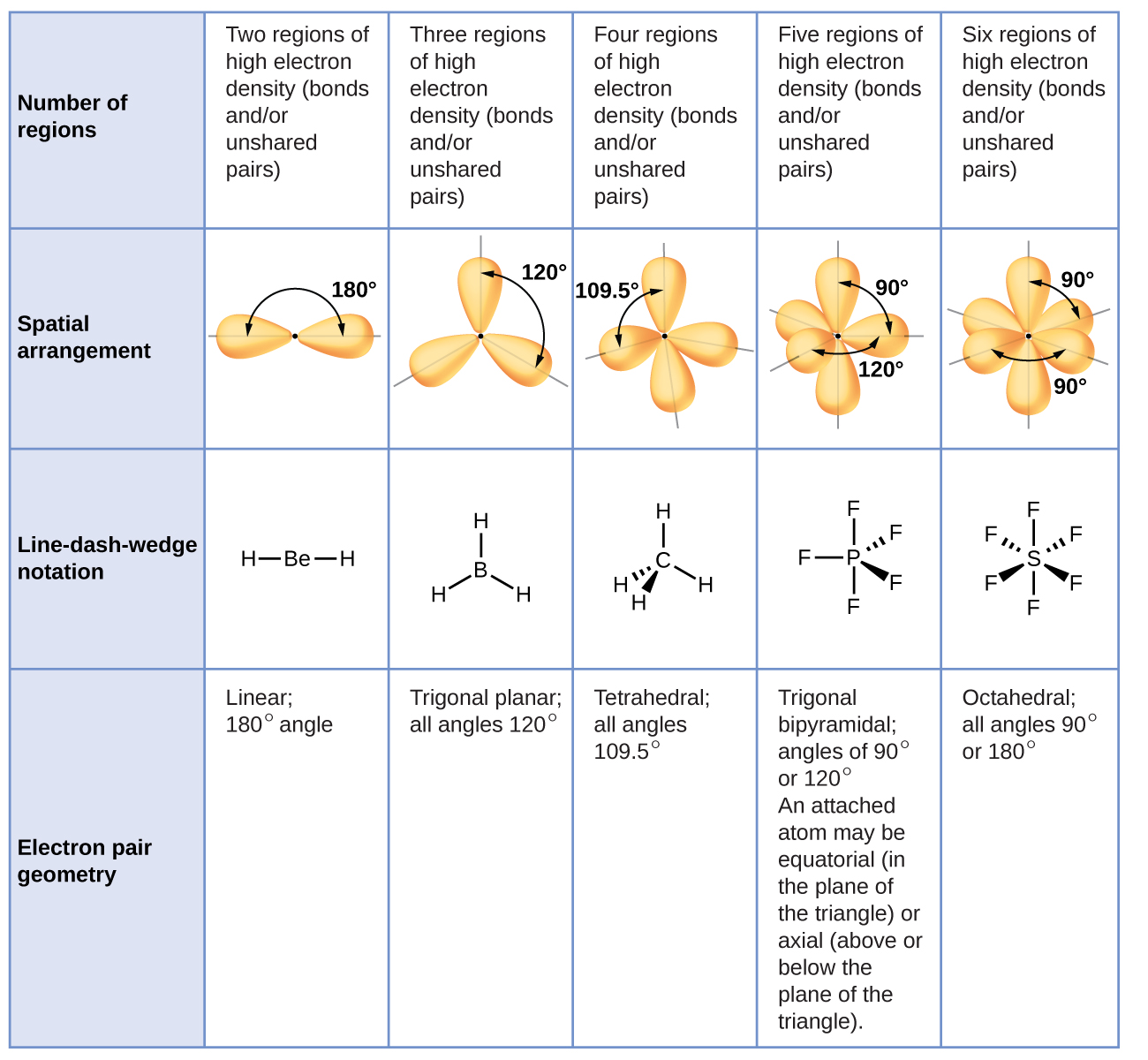
C closer to C because carbon has a lower electronegativity than fluorine. Trigonal pyramidal Tetrahedral O trional planar Bent. A molecule that contains covalent bonds and has an ___ arrangement of electron groups will have an overall ___ polarity which is measured as a dipole ___.
An inadequate model because the bond is ionic Ob centrally located directly between the C and F c. Electron groups could be considered what. Electron groups could be considered as Lone pair electrons and bonded pairs of electrons.
A closer to C because carbon has a larger radius and thus exerts greater control over the shared electron pair. What is the angle between electron groups in the tetrahedral geometry. The table below indicates the Molecular Geometry of the central atom depending on whether the groups of electrons around it are covalent bonds to other atoms or simply lone pairs of electrons.
Which of the following description best describes the type of electrons used to write a Lewis structure. Unequal molecular moment The polarity of a molecule can be expressed in terms of its moment symbol μ which is the product of the partial in the molecule and the between their centers. Start studying the chem flashcards containing study terms like Since enolate formation is an acid-base reaction the stronger the base used for deprotonation the more strongly the equilibrium favors the Which of the following options correctly describe kinetic and thermodynamic enolates and.
Electron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. S p d f. IF the electron groups are covalent bonds.
Option D B. A typical electron configuration consists of numbers letters and superscripts with the following format. Any atom thats an ion D.
Electron groups could be considered which of the following. Closer to C because carbon has a lower electronegativity than fluorine o closer to C because carbon has a larger radius and thus. A lone pair of electrons 3.
Ions of main group elements Noble gases 8A almost completely unreactive due to electron configuration ns2np6 except He 1s2 Main group elements tend to gain or lose electrons to become isoelectronic same valence electron configuration as nearest noble gas. Lone-pair electrons Bonded pairs of electrons. The electron pair in a C-F bond could be considered Select one.
The electron pair in a C-F bond could be considered. Excited electrons core electrons valence. The rate of eh reaction with ethylene is low 106 M-1s-1.
A letter indicates the type of orbital. In such cases an amphiprotic solvent could also be suitable an amphiprotic solvent contains simultaneously an electron donating and electron accepting groups in a single molecule for instance ethanolamine. The electron pair in a C-F bond could be considered A closer to C because carbon has a larger radius and thus exerts greater control over the shared electron pair B closer to F because fluorine has a higher electronegativity than carbon C closer to C because carbon has a lower electronegativity than fluorine.
A double bond 4. To Learn how to Write Electronic Configurations Detailed Explanation Filling of orbital with FAQs Visit BYJUS for detailed. 1 bonded pairs of electrons 2 long-pairs of electrons.
O A double bond A single bond All of the proposed answers Alone pair of electrons Question 2 What is the molecular geometry if you have 3 single bonds and one lone pair around the centralatom. A lone pair of. B closer to F because fluorine has a higher electronegativity than carbon.
If all of the electron groups around a central atom are bonding groups that is there are no lone pairs what is the molecular geometry for three electron groups. A number indicates the energy level The number is called the principal quantum number. A group of electrons can be a single bond double bond triple bond or a lone pair of electrons.
Three or more unshared electrons C.
Comments
Post a Comment